Articolo del Prof. Vittorio Elia in merito alla Ricerca scientifica sulle Alte Diluizioni (EDS – Extremely Diluted Solutions).
Recent studies on the existence of an Exclusion Zone (EZ) at the interface solid/water of a hydrophilic membrane of synthetic nature, Nafion, showed unexpected scenarios involving the properties of water.
This extraordinary liquid shows to have great ability to mutate over its molecular structure in a specific manner according to the chemical nature of the hydrophilic polymer, which has come into contact. The highlight of the complex phenomenology detected is linked to the application of an iterative procedure.
In the early stages of the chemical and physical parameters, applications do not show large variations. Often the numerical values increased by values very close to experimental error of the utilized methodology.
Often these small variations cause the experimenter to abandon the search. The application of the iterative method has allowed us to get into an unknown field today but full of positive and surprising results.
The application of the iterative methodology to water in contact with hydrophilic polymers, suggests that even for the EDS homeopathic dilutions (Extremely Diluted Solutions) we are facing in substantially the same process responsible of the large variations of the physical chemical properties of water.
In the course of these researches, it has been exhaustively demonstrated that the variations of watching you chemical and physical parameters are not due to the chemical nature phenomena dependent on the release of impurities. The polymer most studied in this respect was the Nafion.
The chemical and physical parameters measured and who exhibited considerable variation, up to three orders of magnitude, are: χ electrical conductivity (mS cm-1), pH, density (g cm-3), heat of mixing with acids and / or with bases, UV absorbance, fluorescence microscopy, Atomic Force microscopy, Scanning Electron microscopy and Transmission and Thermogravimetric Analysis.
 In the specific case of Nafion the iterative procedure, adopted most often consists of immersing the polymer film in MilliQ water, wait until it is completely hydrated, perform a delicate mechanical operation on the surface of the polymer with the purpose of removing EZ tufts from the polymer and transferring them to water.
In the specific case of Nafion the iterative procedure, adopted most often consists of immersing the polymer film in MilliQ water, wait until it is completely hydrated, perform a delicate mechanical operation on the surface of the polymer with the purpose of removing EZ tufts from the polymer and transferring them to water.
Leave to dry Nafion. It iterates the procedure immersing the film already in the water changed. It should be emphasized that the variations obtained in the chemical and physical parameters are the same whether they strive pieces of new polymer or if you iterate for months or years the same piece of Nafion.
We believe that the EZ tufts, which have been transported within the liquid, are constituted by aggregates of water molecules. The physical-chemical methodologies that most directly support this working hypothesis are Fluorescence microscopy, AFM Atomic Force, Electronic scanning, SEM and TEM transmittance.
Naturally, the operator have paid much attention to avoiding contamination of the liquid by systematic physical chemical and analytical nature controls. This transaction was made even more necessary when they were first identified the latest chemical and physical parameters that characterize this new type of water we named “perturbed water”, Circular dichroism and fluorescence spectra. In this case, it was necessary to search for organic and biological contaminants as possible pollutants responsible for the two types of spectra, typical of biological macromolecules.
This has made it possible to exclude this hypothesis. Studies on the chemical and physical changes induced by the presence of the synthetic polymer Nafion have been extended to other hydrophilic polymers derived from natural substances and in particular from cellulose derivatives.
The most extensive work on experimental design covered the cotton wool, currently under submission for publication. To highlight the capabilities of liquid water to “recognize” the chemical nature of the polymer just highlight the fact that the Nafion polymer is capable of making the water with which it came into contact in an iterative manner strongly acidic. The cellulose polymer, on the other hand makes it alkaline. Differences and peculiarities!
As part of the positive effects of this research topic to homeopathic dilutions (EDS) is enough to mention the fact that both the perturbed water for contact with hydrophilic polymers that homeopathic dilutions show great similarities in chemical and physical changes.
Recent studies on the existence of an Exclusion Zone (EZ) solid interface / water of a hydrophilic membrane of synthetic nature, Nafion, have highlighted unsuspected scenarios involving the properties of water.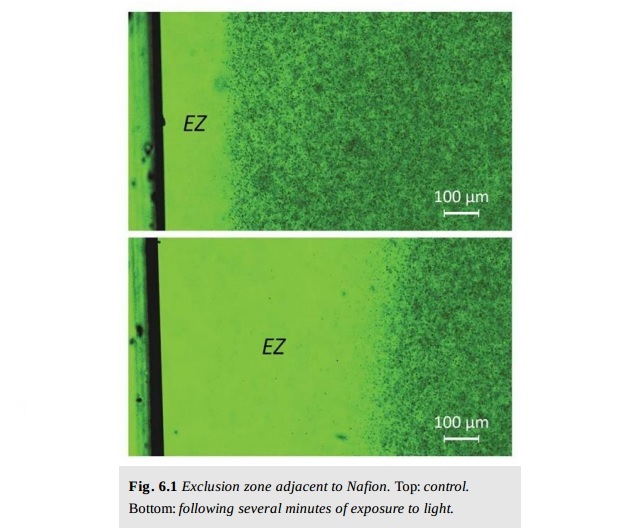
This extraordinary liquid shows to have great ability to mutate over its molecular structure in a specific manner according to the chemical nature of the hydrophilic polymer, which has come into contact. The highlight of the complex phenomenology detected is linked to the application of an iterative procedure.
In the early stages of the applications, do not show significant variations of chemical and physical parameters. Often the numerical values increased by values very close to experimental error of the utilized methodology. Often these small variations cause the experimenter to abandon the search.
The application of the iterative method has allowed us to get into an unknown field today but full of positive and surprising results. We have exhaustively demonstrated that the variations of chemical and physical parameters are not due to the chemical nature phenomena dependent on the release of impurities.
The polymer most studied in this respect was the Nafion. The chemical and physical parameters measured and who exhibited considerable variation, up to three orders of magnitude, are: χ electrical conductivity (mS cm-1), pH, density (g cm-3), heat of mixing with acids and / or with bases, UV absorbance, fluorescence microscopy, Atomic Force microscopy, Scanning Electron microscopy and Transmission and Thermal Gravimetric Analysis.
These variations were obtained by adopting an iterative technique that allows, after a certain number of iterations, to highlight measurable variations and therefore outside of experimental error. In the specific case of Nafion the iterative procedure, adopted most often consists of immersing the polymer film in MilliQ water, wait until it completely hydrated, perform a delicate mechanical operation on the surface of the polymer with the purpose of removing EZ clumps from the polymer and transferring them to water.
Leave to dry Nafion. It iterates the procedure immersing the film already in the water changed. It must emphasized that the variations obtained in the chemical and physical parameters are the same whether they strive pieces of new polymer or whether they have already used for years or months.
The physical-chemical methodologies that most directly support this working hypothesis are Fluorescence (AFM), Atomic Force Electronic Scanning (SEM) and (TEM) Transmittance Microscopies. Of course, the operator has paid much attention to avoiding contamination of the liquid by making systematic checks with the techniques of analytical chemistry. We named this new kind of water “perturbed water”.
Circular Dichroism and Fluorescence spectra are also common to EDS. In this case, it was necessary to search for organic and biological contaminants as possible pollutants responsible for the two types of spectra, typical of biological macromolecules. This has made possible to exclude this hypothesis.
Studies on the chemical and physical changes induced by the presence of the synthetic polymer Nafion have been extended to other hydrophilic polymers derived from natural substances and in particular from cellulose derivatives.
The most extensive work on experimental design covered the cotton wool, currently under submission for publication. To highlight the capabilities of liquid water to “recognize” the chemical nature of the polymer just highlight the fact that the Nafion polymer is capable of making the water with which it came into contact in an iterative manner strongly acidic.
The cellulose polymer, on the other hand makes it alkaline. Differences and peculiarities!
We are therefore faced with a common phenomenon in all types of the perturbed waters examined by us. In essence, a general phenomenon characterizes the water!
All kinds of perturbed waters we studied show a common phenomenon. They exhibit two of the typical spectroscopic properties of the solutions containing biological macromolecules.
The perturbed waters exhibit spectra of Circular Dichroism spectra and emit Fluorescence spectra!
A positive result which could lead to a breakthrough in the study of EDS, typical dilutions of homeopathic pharmacology, well known to be indicated by detractors not informed of the progress of research in this field, such as “fresh water” and therefore unable to interact with the systems organic.
Prof. Vittorio Elia
___________________________________
References
1. Henniker JC (1949). Rev Mod Phys 21: 322.
2. Zheng JM and Pollack GH (2003). Phys Rev E: Stat Nonlinear, Soft Matter Phys 68: 031408.
3. Zheng JM, Chin WC, Khijniak E, Pollack GH (2006). Adv Coll Inter Sci 127: 19.
4. Carrasco J, Hodgson A, Michaelides A (2012). Nature Material 11: 667.
5. Bunkin NF, Ignatiev PS, Kozlov VA, Shkirin AV, Zakharov SD and Zinchenko AA (2013). WATER Journal 4: 129.
6. Tychinsky V (2011). WATER Journal 3: 95.
7. Gun’ko VM, Turov VV, Bogatyrev VM, Zarko VI, Leboda R, Goncharuk EV, Novza AA, Turov AV, Chuiko AA (2005). Adv Coll Inter Sci 118: 125.
8. Mártonfalvi Z and Kellermayer MSZ (2008). Nanomechanics of exclusion-zone water. Proceedings of 2008 Meeting on Physics, Chemistry and Biology of Water, Mount Snow, Vermont, USA.
9. Chai B, Mahtani AG, Pollack GH (2012). Contemp Mater 3: 1-12.
10. Germano R, Del Giudice E, De Ninno A, Elia V, Hison C, Napoli E, Tontodonato V, Tuccinardi FP, Vitiello G (2013). Key Engineering Materials 543: 455.
11. Liu ZQ, Zhang GC, Li YJ, Jiang SR (2012). Phys Rev E 85: 036314.
12. Shirsavar R et al. (June 9, 2008). Liquid motor revs up. Scientific American.
13. Chai B, Yoo H, Pollack GH (2009). J Phys Chem B 113: 13953.
14. Zheng JM, Wexler A, Pollack GH (2009). J Colloid Interface Sci 332: 511.
15. Chai B and Pollack GH (2010). J Phys Chem B 114: 5371.
16. Yoo H, Paranji R, Pollack GH (2011). J Phys Chem Lett 2: 532.
17. Yoo H, Baker DR, Pirie CM, Hovakeemian B, Pollack GH (2010). Characteristics of water adjacent to hydrophilic interfaces. In: D. LeBihan, H. Fukuyama, editors. Water, the Forgotten Biological Molecule. Pan Stanford Publishing Pte. Ltd.
18. Nhan DT and Pollack GH (2011). Int J Des Nat Ecodyn 6: 139.
19. Nagornyak E, Yoo H, Pollack GH (2009). Soft Matter 5: 3850.
20. Chai B, Zheng J, Zhao Q, Pollack GH (2008). J Phys Chem A 112: 2242.
21. Zheng JM, Pollack GH (2006). Solute exclusion and potential distribution near hydrophilic surfaces. In: Water and the Cell. Springer, Netherlands.
22. Pollack GH, Clegg J (2008). Unexpected linkage between unstirred layers, exclusion zone, and water. In: Phase Transitions in Cell Biology. Springer, New York.
23. So E, Stahlberg R, Pollack GH (2011). Exclusion zone as intermediate between ice and water. In: Water and Society. WIT Press, Southampton.
24. Elia V, Napoli E, Niccoli M (2013). J Therm Anal Calorim 112: 937.
25. Elia V, Ausanio G, De Ninno A, Gentile F, Germano R, Napoli E, Niccoli M. (2013). WATER Journal 5: 16.
26. Elia V, Lista L, Napoli E, Niccoli M (2014). J Therm Anal Calorim 115: 1841.
27. Carrasco J, Klimeš J, Michaelides A (2013). J Chem Phys 138: 024708.
28. Ferri N, DiStasio RA, Ambrosetti A, Car R, Tkatchenko A (2015). Phys Rev Lett 114: 176802.
29. Robinson RA and Stokes RH. (1959). Electrolyte Solutions. Dover Publications Inc., Mineola, New York.
30. Buch V, Tarbuck T, Richmond GL, Groenzin H, Li I, Shultz MJ (2007). J Chem Phys 127: 204710.
31. Noguchi H, Hiroshi M, Tominaga T, Gong JP, Osada Y, Uosaki K (2008). Phys Chem Chem Phys 10: 4987.
32. Faraudo J (2011). Current Opinion in Colloid & Interface Science 16: 557.
33. Del Giudice E, Spinetti PR, Tedeschi A (2010). Water 2: 566.
34. Del Giudice E, Tedeschi A, Vitiello G, Voeikov V (2013). J Phys: Conf Ser 442, 012028.
35. Del Giudice E, Voeikov V, Tedeschi A, Vitiello G (2014). The origin and the special role of coherent water in living systems. Chapter 5 in: Fields of the Cell. D. Fels, M. Cifra and F. Scholkman, editors. Research Signpost, Kerala, India.
36. Roy BN (2002). Fundamentals of classical and statistical thermodynamics. Wiley, West Sussex, UK.
37. Onsager L (1936). Electric Moments of Molecules in Liquids. J Amer Chem Soc 58: 1486-1493.
38. Kirkwood JG (1939). The Dielectric Polarization of Polar Liquids. J Chem Phys 7: 911-918.
39. Sivasubramanian S, Widom A, Srivastava YN (2005). Physica A 345: 356.
40. Martin DR and Matyushova DV (2008). Microscopic fields in liquid dielectrics. J Chem Phys 129: 174508.
41. Feynman RP, Leighton RB, Sands M. (1964). Feynman Lectures on Physics. Vol. 2, chap. 11. Addison Wesley.
42. Geiger R and Klapp SHL (2013). Dimensionality Effects in Dipolar Fluids: A Density Functional Theory Study. J Mod Phys 4: 401-408.
43. Del Giudice E and Vitiello G (2006). Phys Rev A 74: 022105.
44. Blasone M, Jizba P, Vitiello G (2011). Quantum theory and its macroscopic manifestations. Imperial College Press, London.
45. Groh B and Dietrich S (1994). Phys Rev Lett 72: 2422.
46. Wei D and Patey GN (1992). Phys Rev Lett 68: 2043.
47. Klapp SHL (2005). Dipolar fluids under external perturbations. J Phys Condens Matter 17: R525-R550.
48. Brändas EJ (2009). Complex Symmetry, Jordan Blocks and Microscopic Self-organization. In: Self Organization of Molecular Systems. Eds. N. Russo, V.Y. Antonchenko, E.S. Kryachko. NATO Science for Peace and Security Series A: Chemistry and Biology: pp 49-87 and references therein.
49. CRC Handbook of Chemistry and Physics (2001). CRC Press, Cleveland, USA.
50. Weis JJ (2003). J Phys Condens Matter 15: S1471.
51. Leroy F, dos Santos DJVA, Müller-Plathe F (2009). Macromol Rapid Commun 30: 864.
52. Del Giudice E, Preparata G, Vitiello G (1988). Phys Rev Lett 61: 1085.
53. Yinnon TA and Elia V (2013). Int J Mod Phys B 27: 1350005.
54. Fenn EE, Wong DB, Fayer MD (2009). Proc Natl. Acad Sci USA 106: 15243.
55. Liu ZQ, Li YJ, Zhang GC, Jiang SR (2011). Phys Rev E 83, 026303.
56. Liu ZQ, Jiang SR, Yinnon TA, Kong XM, Li YJ (2016) Effects of interfaces on dynamics in micro-fluidic devices: slip-boundaries’ impact on rotation characteristics of polar liquid film motors. To be submitted.
57. Nakamura Y and Ohno T (2011). Phys Chem Chem Phys 13: 1064.
58. Arani R, Bono I, Del Giudice E, Preparata G (1995). Int J Mod Phys B 9, 1813.
59. Ho MW (2014). WATER Journal 6: 1.
60. Di Stasio RA, von Lilienfeld OA, Tkatchenko A (2012). Proc Natl Acad Sci USA 109: 14791.
61. Jones AP, Crain J, Sokhan VP, Whitfield TW, Martyna GJ (2013). Phys Rev B 87: 144103.
62. Sivasubramanian S, Widom A, Srivastava YN (2001). Physica A 301, 241.
63. Del Giudice E, Mele R, Preparata G (1993). Mod Phys Lett B 7: 1851.
64. Del Giudice E, Galimberti A, Gamberale L, Preparata G (1995). Mod Phys Lett B 9: 953.
65. Bono I, Del Giudice E, Gamberale L, Henry M (2012). Water 4: 510.
66. Yinnon CA and Yinnon TA (2009). Mod Phys Lett B 23: 1959.
67. Röntgen WC (1892). Ann Phys 281: 91.
68. Mishima O and Stanley HE (1998). Nature 396: 329.
69. Poole PH, Sciortino F, Essmann U, Stanley HE (1992). Nature 360: 324.
70. Stokely K, Mazza MG, Stanley HE, Franzese G (2010). Proc Nat Acad Sci 107: 1301.
71. Huang C et al. (2009). Proc Natl Acad Sci USA 106: 15214-15218.
72. Taschin A, Bartolini P, Eramo R, Righini R, Torre R (2013). Nature Commun 4:2401.
73. Yinnon TA and Liu ZQ (2015) WATER Journal 7: 33. idem 48; idem 70.
74. Preparata G (1995). QED Coherence in Matter. World Scientific, Singapore, New Jersey, London, Hong Kong.
75. Del Giudice E, Fuchs EC, Vitiello G (2010). WATER Journal 2: 69-82.
76. Nakamoto K (1997). Infrared and Raman spectra of inorganic and coordination compounds. Wiley, West Sussex, UK.
77. De Ninno A, Congiu Castellano A (2011). J Molec Structure 1006: 434.
78. De Ninno A, Congiu Castellano A, Del Giudice E (2013). J Phys: Conference Series 442: 012031.
79. Pershin SM, Bunkin AF, Lukyanchenko VA, Nigmatullin RR (2007). Laser Phys Lett 4: 809.
80. Arakawa M, Kagi H, Fukazawa H (2009). Astrophysical Journal Supplement Series 184: 361.
81. Grimmett G (1999). Percolation. Grundlehren der mathematischen Wissenschaften, Vol. 321. Springer.
82. Vrbka L and Jungwirth P (2005). Phys Rev Lett 95: 148501.
83. Gross GW, Wong PM, Humes K (1977). J Chem Phys 67: 5264.
84. Cogoni M, D’Aguanno B, Kuleshova LN, Hofman DWM (2011). J Chem Phys 134: 204506.
85. Iitaka T (2010). arXiv:1007.1792v1 cond-mat.mtrl-sci.
86. Yokono T, Shimokawa S, Yokono M, Hattori H (2009) WATER Journal 1: 29.
87. Toyama N, Kohno JY, Kondow T (2006). Chem Phys Lett 420: 77.
88. Amjadi A, Shirsavar R, Radja NH, Ejtehadi MR, (2009). Microfluid Nanofluid 6: 711.
89. Capolupo A, Del Giudice E, Elia V, Germano R, Napoli E, Niccoli M, Tedeschi A, Vitiello G (2014). Int J Mod Phys B 28: 1450007.